Acryl-D01: AI-Powered Medical Device Supporting Depression Diagnosis, Gaining Attention for Its Potential in Psychiatry
The AI-powered medical device "Acryl-D01," designed to assist in diagnosing depression by analyzing patient interview records, is garnering attention for its potential to enhance the efficiency of psychiatric care and successfully integrate into clinical practice.
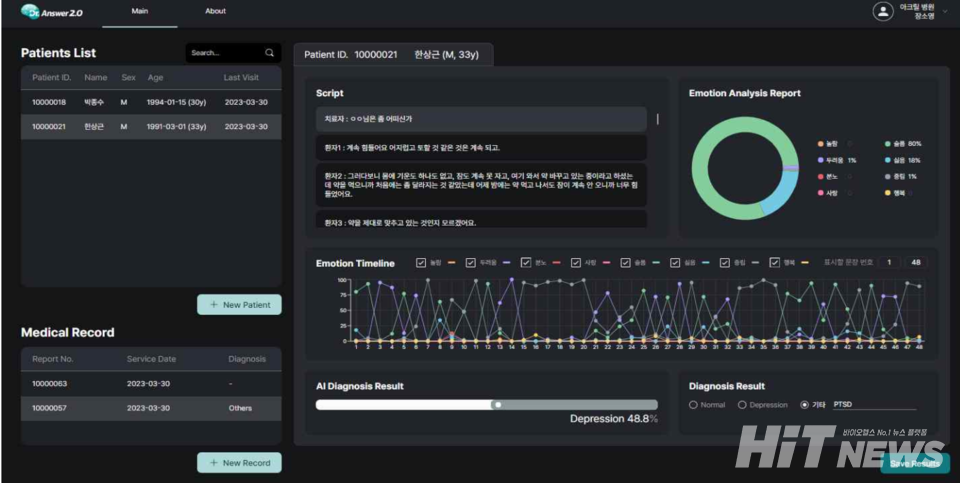
On the 20th, the Ministry of Food and Drug Safety approved Acryl-D01, a depression probability indication software medical device developed by Acryl (CEO Jin Park). Acryl-D01 is a Class II medical device that utilizes artificial intelligence to analyze interview records between medical professionals and patients, providing a quantified probability of depression.
The device quantifies the likelihood of depression based on patient interview records using AI technology, assisting physicians in their diagnostic processes.
A representative from Acryl's AI Business Support Team explained, "Acryl-D01 was developed for use by both psychiatrists and non-psychiatric physicians. Psychiatrists typically record patient interviews based on the depression monitoring and evaluation standards outlined in depression treatment guidelines. Acryl-D01 extracts emotional indicators from these records and calculates the probability of depression using AI algorithms."
The representative added, "Non-psychiatric physicians can also use Acryl-D01 effectively in clinical settings when assessing patient anxiety or depression levels using depression scales."
The company expects Acryl-D01, as an AI medical device designed for use with standardized interviews conducted according to clinical guidelines, to significantly improve diagnostic efficiency.
During research and validation, the evaluation process, which usually takes about 10 minutes, was reduced to just 10 seconds. The representative noted, "While the development phase relied on manually inputted interview records, the commercial version will incorporate speech-to-text technology for greater efficiency."
However, the company has yet to decide whether to pursue inclusion in the national health insurance system for reimbursement or to position the device as a tool for improving diagnostic efficiency.
The representative stated, "This product was developed in collaboration with Seoul St. Mary’s Hospital as part of the Ministry of Science and ICT and the National IT Industry Promotion Agency (NIPA)'s AI precision medicine project, 'Dr. Answer 2.0.' It began with academic and social objectives rather than commercial considerations, so the business model is not yet fully defined. Once the final report is completed early next year, we will move forward with commercialization."
The representative also noted, "Acryl-D01 is the model name assigned during the approval process. For commercialization, it will be marketed under the name 'Esther Deprex.' The name 'Esther' honors Korea's first female physician, Dr. Esther Park (1877-1910), symbolizing an AI that offers compassionate and informative guidance."